Cantly under highly oxidizing conditions 250 to 500 mV. Dear Shabir Ahmed I have prepared for you a summary about the EFFECT OF REDOX POTENTIAL ON MICROBIAL GROWTH.
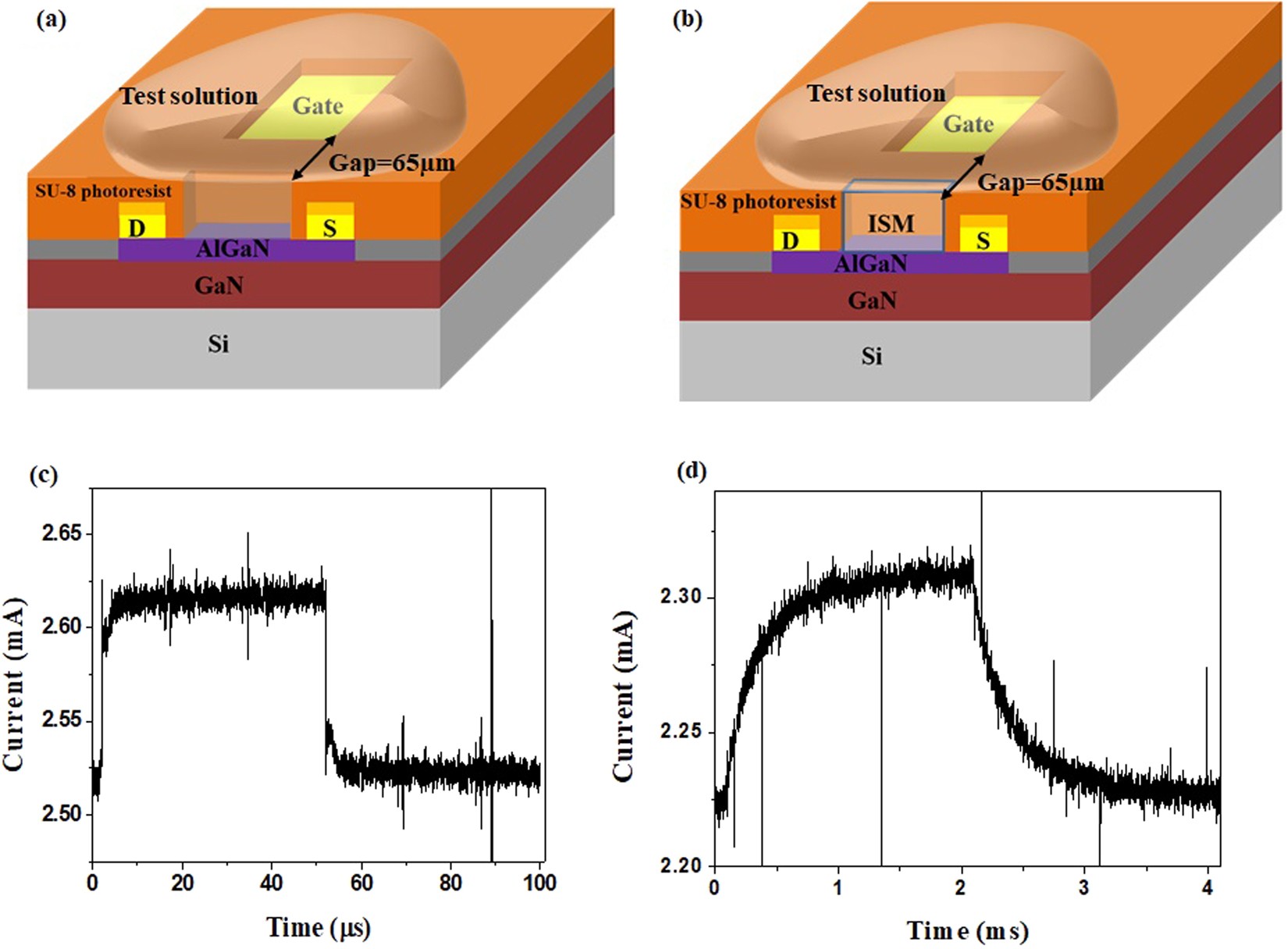
High Field Modulated Ion Selective Field Effect Transistor Fet Sensors With Sensitivity Higher Than The Ideal Nernst Sensitivity Scientific Reports
An effective concentration of 1 molL for each aqueous species or a species.

Effect of redox potential of tin. Results showed that the soil suspension under continuous oxygen aeration for 21 days resulted in increases of redox potential from 290 to 440 mV and. The regional current system in the Gulf is characterized by northward flowing surface waters and a southward flowing bottom waters except near the shore where it has a gyratory character. The metal chelates ZnEDTA ZnDTPA CuEDTA and CuDTPA were reacted with soil suspensions incubated at six different redox potentials 200 100 0 100 300 and 500 mV and at pH 7 for reaction periods varying from 2 to 192 hours.
The redox potential has a very significant effect on the stability of all four metal chelates studied. The sub-millimolar tinI V and tinII concentrations make the electrochemical cell poorly buffered and probably extremely oxygen sensitive because even traces of oxygen could significantly change the relative concentrations of SnIV and thus the cell potential. V 3 V 2 reduction by reducing its peak potential separation from 1011 to 589 mV owing to the deposition of tin nanoparticles in its vicinity.
Effect of tributyl tin chloride on the change in oxidation-reduction potential of the environment in the growth of the river microflora Nauchnye Doki Vyss Shkoly Biol Nauki. The specific effect of tin on the valence equilibrium of iron in the surface layers of float-glass. Redox potential E h is the measurement of the tendency of an environment to oxidize or reduce substratesAn aerobic soil which is an oxidizing environment has an E h of800 mV.
The adsorbed tin II chloride inhibits the oxidation of the bulk tin II chloride and therefore shifts the potential for oxidation to more positive values. Cyclic voltammetry reflects the excellent improvement in reaction kinetics particularly for the anode half-reaction ie. To investigate the effect of the redox potential on the methane-producing activity of M.
The influence of redox potential and pH on arsenic speciation and solubility was studied in a contaminated soil. Oxygen is found in soils at a redox potential of about800 mVWhen soil is placed in a closed container oxygen is. The redox potential of the glass matrix affects the equilibrium between difference valence forms of iron much less than additives correcting spectral characteristics of glass.
Standard electrode potential data page The data values of standard electrode potentials E are given in the table below in volts relative to the standard hydrogen electrode and are for the following conditions. 47 ration lead to a rise in the salinity causing a steep salinity gradient along the Gulf. This report elucidates the distinctions of redox properties between two uptake hydrogenases in Escherichia coliHydrogen uptake in the presence of mediators with different redox potential was studied in cell-free extracts of E.
Therefore the standard potential determined under these conditions. The effects of redox potential and primary oxidant type and concentration on metal release corrosion rate and corrosion scale properties of distribution system pipe material lead copper and iron are poorly understood. The main difficulties of making an accurate determination of the redox state of glass are noted.
Both redox potential and pH were found to greatly affect heavy metal solubility in the soil. As has been pointed out in the preceding paper 20 studying the effect of the concentration of a dithionite- reduced electron donor above pH 75 is in general no problem. Thermautotrophicus excluding the effect on growth a short-term BES experiment 7 h using resting cells high biomass was performed.
A temperature of 29815 K 2500 C. The dynamics of redox potential were induced by changing the water -table depth in a lab -. The Effect of Redox Potential on the Stability of Some.
A single-chamber electrolysis cell was used to measure the methane production under potentiostatic conditions with a fixed redox potential. Redox or oxidoreduction potential is defined as the sum of all the oxidizing dissolved oxygen free radicals hydrogen peroxide some oxidized metal ions and reducing some vitamins some reduced metal ions thiol-containing molecules hydrogen couples found. The effect of 3d-electron configuration entropy on the temperature coefficient of redox potential in Co1zMnz Prussian blue analogues 著者英 Hiroki Iwaizumi Yusuke Fujiwara Yuya Fukuzumi Yutaka MORITOMO journal or publication title Dalton transactions volume.
An anaerobic soil which is a reducing environment has a negative E h which can reach 300 mV. Redox potential is directly related to many drinking water processes including disinfection iron and manganese removal and corrosion of distribution system materials. Coli mutants HDK103 and HDK203 synthesizing hydrogenase 2 or hydrogenase 1 respectivelyBoth hydrogenases mediated H 2 uptake in the presence of high-potential.
Very little leached at an ORP of 50 mV or less. Effect on metals leaching. The effect of some additives on the equilibrium of valence forms of iron is investigated.
Adshelpatcfaharvardedu The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative Agreement NNX16AC86A. Maritima was shown to significantly decrease the redox potential Eh of the culture medium down to about 480 mV as long as glucose was available. The electrocatalytic effects of tin cause a significant improvement in key performance parameters of voltage efficiency energy.
The redox behavior of tin in molten glass is of great importance for glass technology. Chromium leaching increased signifi-. To assess the mobilities of Pb Cd and Zn from a contaminated soil the effects of redox potential and pH value on metal solubilities were investigated.
At higher soil redox levels 500-200 mV arsenic solubility was low and the major part 65-98. Maritima cultures during the stationary growth phase led to a drastic reduction in glucose consumption rate. Alterations in the oxidation state of arsenic and influenced by redox potential and pH greatly affected its solubility in soil.
Under our experimental conditions we found that N2O emissions followed closely the changes in redox po tential. However once the adsorbed tin II chloride is oxidized the dissolved tin II chloride is oxidizable at less positive potentials. Redox potential induced by changes in the water level affect GHG emis-sions from agricultural soil.
Research showed that redox potential has a significant. The effects of redox potential and the proton and electron donor concentration on the activity are of particular in- terest. Addition of oxygen into T.
When grown anaerobically on glucose T. It is demonstrated that tin and carbon more significantly than fluorine reduce iron. This is especially due to the float glass process which in the past few years proved to be a versatile process enabling the processing of glass melts of various compositions.
Arsenic vanadium lead and iron leaching rates all increased signifi-.

Catalytic Effects In The Cathode Of Li S Batteries Accelerating Polysulfides Redox Conversion Sciencedirect

Effects Of In Situ Bismuth Catalyst Electrodeposition On Performance Of Vanadium Redox Flow Batteries Sciencedirect
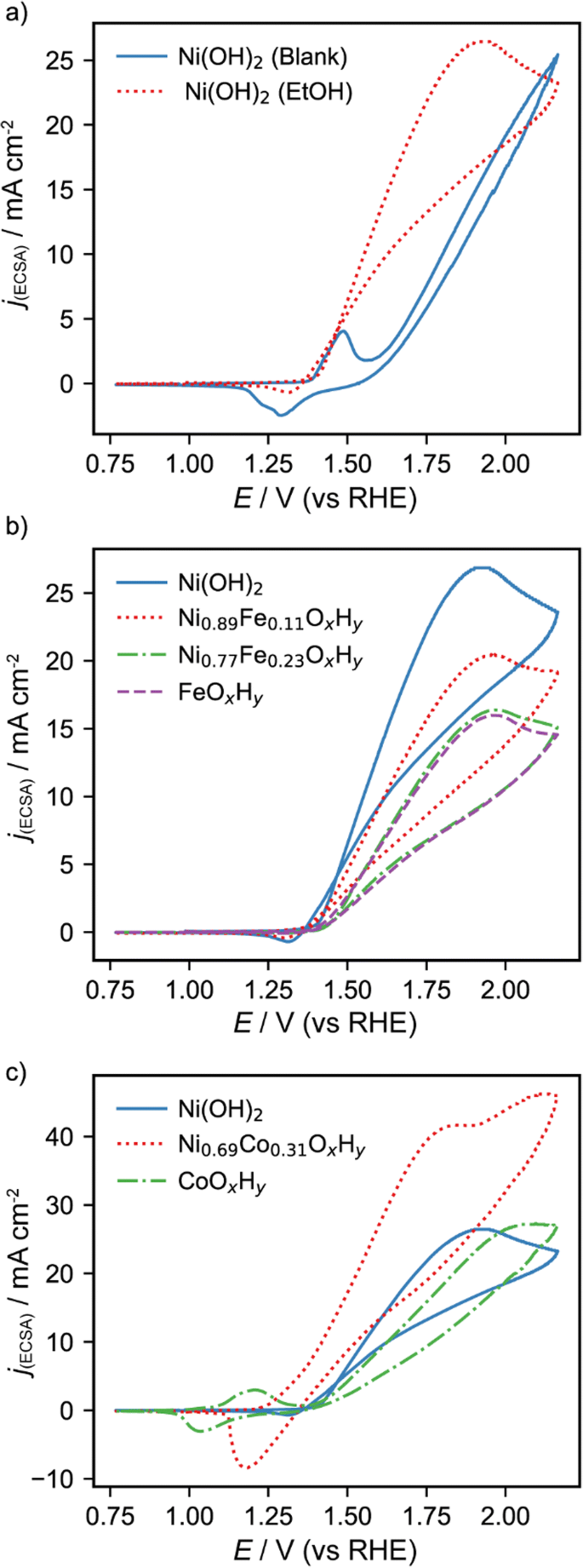
Effects Of Incorporated Iron Or Cobalt On The Ethanol Oxidation Activity Of Nickel Oxy Hydroxides In Alkaline Media Springerlink

Pdf Impact Of Redox In Industrial Glass Melting And Importance Of Redox Control

Effect Of The Oxidation State And Morphology Of Snox Based Electrocatalysts On The Co2 Reduction Reaction Springerlink
Tidak ada komentar