The article also describes the methods of corrosion testing of coatings. Galvanic Corrosion and Dissimilar Metals Factors Affecting Galvanic Corrosion There are a number of factors that determine the occurrence and severity of galvanic corrosion.

Tin Plating Company Corrosion Resistant Plating Services Electroplating Nickel Plating Tin
The contact resistance increases in accordance to the thickness of tin oxidation film 3.
Factor that influence corrosion resistance for tin plating. Moreover the wear track depth did not exceed the coating thickness for TiN. The susceptibility of tin-plated contacts to fretting corrosion is a major limitation for its use in electrical connectors. Electrochemical porosity measurements which are essential for the estimation of the corrosion resistance of coated components were made in 1 N sulfuric acid solution at ambient temperature.
A variety of factors influence the. The following are the most significant factors to consider for a corrosion-protective finish. There is unlikely to have the present of.
The results showed that heating power and time of reflowing had effects on the corrosion resistance of tinplate. The factors evaluated were the thickness of the coating the thickness and the type of the intermediate layer the composition of the coating and the number of heterogeneities in the coating. Figure 14 illustrates that by.
Zinc plating is primarily used to increase corrosion resistance on smaller metallic parts such as nuts bolts and screws. High nickel content results in a much better resistance to stress corrosion cracking than the standard austenitic grades. Nickel Nickel is a strong lustrous metal that is often used as a base coat prior to plating with a precious metal such as gold or silver.
Lower levels of phosphorous allow higher solderability and magnetism. Plane geometry and subjected to fretting under gross-slip conditions. Corrosion-resistant material for a particular environment may just not make a suitable fastener.
The corrosion behavior of TiN-coated stainless steel is controlled by two factors. The PREN factor is defined as. In consumer electronic circuit application these interconnects are exposed in atmospheric condition.
Removal of tin coating in many. Precious metal coatings seal off the substrate from the environment creating a smooth protective finish that prevents water and oxygen from reaching the base material. Higher levels of phosphorous enhance hardness and corrosion resistance.
However titanium can be easily corroded by HF or some concentrated. It reviews the influence of corrosion on immersion tin coating tin-cadmium alloy coatings tin-cobalt coatings tin-copper coatings tin-lead coatings tin-nickel coatings and tin-zinc coatings. Corrosion occurs because of the defects in structure and it is aggravated by the poor adhesion strength of TiN coatings.
Zincs primary benefit is that it offers excellent protection against corrosion. N Position on the galvanic series scale of the fastener and materials to be joined. The corrosion area of tin layer surface was gradually reduced with the decrease of quenching water temperature and its corrosion resistance.
One of the most widely used methods for TiN coating deposition is Physical Vapor Deposition PVD. A PREN factor of 35 indicates that the material has good resistance to warm sea water and other high chloride containing environments. Tin electrodeposits are primarily used for functional purposes such as providing a level of protection or corrosion resistance to a range of items.
Coatings is TiN due to excellent corrosion resistance high conductivity a similar water contact angle as graphite wide industrial feasibility low cost and extensive research as well. As it has been reported in literature the DLC coating presented better wear resistance than TiN coating in this kind of tests. Tin is extremely cost-effective and is commonly applied to a preliminary coating of copper.
The percentage of phosphorous in the solution can vary between 2 and 14. N Tensile and fatigue strength. The factors involved in the study are load plating thickness amplitude temperature.
Interlayer coatings could reduce the defect density consequently improve the corrosion resistance. The variation in contact resistance as a function of fretting cycles and the time to reach a threshold value 100mO of contact resistance enables a better understanding of the influence of various factors on the fretting corrosion behaviour of tin-plated contacts. Higher temperature accelerates the diffusion of oxygen due to its influence on the solubility of oxygen in electrolyte.
It also shows high resistance to a wide range of environments. Sometimes the presence of film porosity lowering the density of film than the bulk materials causes for more corrosion in coating. N Special design considerations.
Basic factors affecting the choice of corrosion resis-tant threaded fasteners are. In electroless plating a nickel phosphorous alloy is used. 22 Tin Thickness Tin thickness has significant impact on the increased contact resistance by fretting corrosion.
The present paper evaluates the influence of a variety of factors such as fretting amplitude track length frequency temperature humidity normal load and current load on the fretting corrosion behaviour of tin-plated. Some couples will cause corrosion more quickly than others depending on the materials that are coupled the environment and the design. The DLC films are normally smooth due to their amorphous character and.
Regulating the amount of phosphorous will have an impact on the electroless nickel coatings ability to ward off corrosion in specific environments. Rate of galvanic corrosion tends to increase with the rising temperature. Through one-factor experiment the effects of heating power heating time quenching water temperature on the corrosion resistance of tinplate were investigated.
Conductivity heat or wear resistance lubrication or solder ability and hence to make possible the use of cheaper substrate metals or plastics covered to give essential metallic. Surface treatments which will impart corrosion resistance or particular physical or mechanical properties to the surface eg. The general properties and corrosion resistance of tinplate are summarized.
Decreases the total area of asperities and causes the contact resistance to increase rapidly. Improving the mechanical properties and increasing the corrosion and wear resistance of different metals and alloys. 1 studied the influence factors of the tin plated contacts with regard to fretting corrosion.
Tin Plating Vs Silver Plating. 17 indicated that the electrical contact. The synergetic effect of packing factorthickness of TiN which is related to the structure of a hard coating and the interfacial stress which is related to the adhesion of TiN coating.
For instance a low-phosphorous coating will provide the best corrosion protection in alkaline environments while a high-phosphorous content is better suited for acidic environments. The wear rate for TiN coating was reduced about five times with respect to the untreated sample as it is shown in Table 2. The further apart the coupled.
Titanium can withstand corrosion attack by dilute sulfuric and hydrochloric acids chloride solutions and most organic acids.
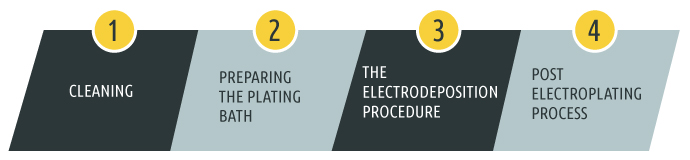
The Tin Plating Process A Step By Step Guide Sharretts Plating

Tin The Forgotten Foot Soldier Of The Energy Transition Wood Mackenzie

Pdf The Effect Of Zinc Tin And Lead Coating On Corrosion Protective Effectiveness Of Steel Reinforcement In Concrete

Pdf Internal Corrosion In Tinplate Cans
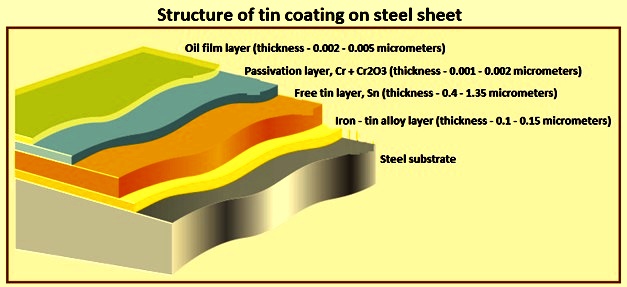
Tidak ada komentar