Electrochemical migration ECM which is a form of corrosion significantly compromises the corrosion reliability of electronic devices 1-3. Open Yenka Model 1.

Enhanced Electrolytic Nitrate Reduction Utilizing A Three Dimensional Electrolysis Reactor Packed With Activated Carbon And Foamed Copper Environmental Engineering Science
The Electrolysis of Aqueous Sodium Chloride.

Electrolysis of tin nitrate. N O X 3 X N O X 2 1 2 O X 2 e X and cathodically by the electrochemical reduction of nitrate ions into nitrite ions. This process occurs when two oppositely biased and closely spaced electrodes are connected by an aqueous electrolyte. Electrolysis of an aqueous solution may generate products other than oxygen or hydrogen if the electrolyte contains ions that are more easily oxidized or more easily reduced than water molecules.
At the high voltage used in this experiment and the low concentration of lead nitrate in solution there also is stronger oxidation of the anode with formation of tinIV and leadIV compounds. 2H 2 O l 2Cl aq H 2 g Cl 2 g 2OH aq E cell 2186 V. Electrolysis of a sulfuric acid solution.
Mechanisms have been proposed to explain the ECM of tin in the TELs with various nitrate concentrations. The solid lines which are shown in Fig. An aqueous solution of a compound is a solution produced when the compound is dissolved in water.
Electrolysis of cobaltII nitrate. Remember nitrate is a good oxidizer used in pyrotechnics and it easily oxidizes weaker reductors like sulphur diverse metal powders etc. The overall reaction is then.
So imagine what happens if molten K is mixed with molten KNO3. A r of Ag 108. However in Chemistry pretty well all general trends and rules have ano.
During this electrolysis reaction hydrogen ions should be reduced at the cathode not Zn2 ions because zinc is more reactive than hydrogen and the rule is that the least reactive ions should be reduced. If the solution contains only one material like the electrolysis of molten sodium chloride it is a simple matter to determine what is oxidized and what is reduced. Heated to 60 C it melts in its water of crystallization.
The simulation shows the mass deposited at the cathode and the mass lost at the anode and animations at the particle level of what occurs at each electrode. Tin Man Electrolysis Demonstration Worksheet The original conducting solution contains tinII chloride SnCl 2. The electrolysis of silver nitrate solution using a silver anode.
During electrolysis of the tin II chloride the tin ions are reduced around the cathode and crystallize into metal tin and Chlorine ion Oxidize around Anod. This is an example of a case where you are using an electrode which gets chemically involved in the reaction. Draw two sketches representing your observations during the first and second parts of this demonstration.
The Electrolysis Computer Simulation provides an opportunity to construct and experiment with several metal-metal electrolytic cells. When aqueous solutions of ionic compounds are electrolyzed the anode and cathode half-reactions may involve the electrolysis of either water species H 2 O H OH or solute species the cations and anions of the compoundAs an example the electrolysis of aqueous sodium chloride could involve either of these two anode reactions. Electrolysis can be carried out on aqueous solutions of less than 1cm3 in volume.
On the other hand the FE decreases almost linearly in 100 mM of nitrate reaching 25 after 90 min of electrolysis. 1 were derived from the. The electrolysis is carried out in the well of a.
Electrolysis of aqueous copper II nitrate. The potential range is limited anodically by the electrochemical oxidation of nitrate ions into nitrogen dioxide and oxygen. Since solid pieces of copper are involved C u must be considered in the reduction potential as well.
B hydrogen ions H and hydroxide ions OH from the partial dissociation of water molecules. AgAgCl in a solution containing 05 mol L 1 NaNO 3 1 mol L 1 NaOH. The first thing to do is to work out how many coulombs of electricity flowed during the electrolysis.
The products of the electrolysis reaction are tin0 and tinIV chloride. Electrolysis is done in solutions which contain enough ions so current can flow. This reduces waste reduces risk and can be achieved in minutes.
In order to get more information for the reactions of the intermediates of nitrate reduction an electrolysis experiment was initially performed at 22 V vs. F 965 x 10 4 C mol-1 or 96500 C mol-1 if you prefer. Add the potassium nitrate solution to the beaker and close the switch.
Lead-fluosilicate may be crystallized in very soluble brilliant crystals resembling those of lead-nitrate and containing four molecules of water of crystallization with the formula PbSiF64H2O. 3696 Electrolysis of silver nitrate solution with an OHP See. Calculate the mass of silver deposited at the cathode during the electrolysis of silver nitrate solution if you use a current of 010 amps for 10 minutes.
Potentiometers Commercial Observe the electrolysis of silver nitrate solution under a microscope or with an overhead projector. Thus sodium hydroxide can be obtained by evaporating the water after the electrolysis is complete. Watch the display for about 30 seconds then decide if any metal is being deposited on the.
If you electrolyse silver nitrate solution using silver as the anode silver is deposited on whatever material the cathode is made of as you would expect. This salt dissolves at 15 C. In this experiment the anode is a tinlead soldering alloy with 40 lead and 60 tin.
TinII chloride also known as stannous chloride is a white crystalline solid with the formula Sn Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in electrolytic baths for tin-platingTinII chloride should not be confused with the other chloride of tin. The electrolysis of aqueous silver nitrate AgNO 3 for example produces oxygen at the anode and silver metal at the cathode. At the cathode if any K-metal is formed at all youll get an immediate redox-reaction between the nitrate ions and the K-metal.
Analysing the Electrolysis of Aqueous Solutions. There are two copper blocks sitting in the C u N O X 3 X 2 a q solution a battery is attached onto both of them providing enough energy to start the reaction. The FE is about 100 in the first 120 min of electrolysis in 1 M of nitrate and then decreases to 78 at 210 min.
N O X 3 X 2 e X N O X 2 X O X 2. During the electrolysis of an aqueous solution of a. As the reaction proceeds hydroxide ions replace chloride ions in solution.
Answer 1 of 2. All the experiments below used approximately 01M solutions except for silver nitrate and tin II chloride which were about 005M. The electrolysis variables starter model.
1 shows the concentration profile of nitrate and of the products of the electrolysis. An aqueous solution of a compound contains a anions and cations of the compound. In 28 per cent of its weight of water making a sirupy solution of 238 sp.
Use a 2 mm wide strip silver cathode a platinum anode a 2M solution of silver nitrate and a power source less than 2 V. Gases collected at the electrodes are tested. An electric current is passed through a sulfuric acid solution.
So there will also be oxidation of tin. Choose metal electrodes electrolyte current and time. Electrolysis of a calcium nitrate solution produces oxygen at the anode and hydrogen at the cathode.
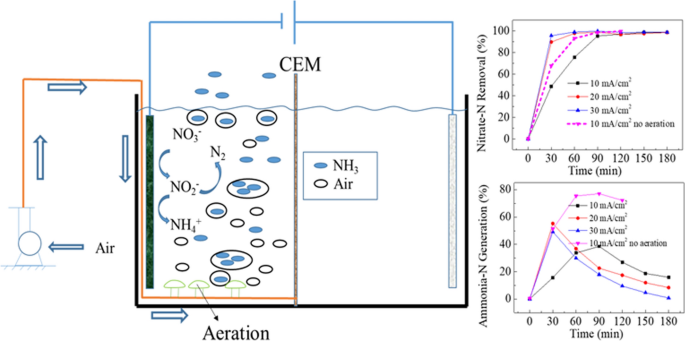
Cu Ni And Multi Walled Carbon Nanotube Modified Graphite Felt Electrode For Nitrate Electroreduction In Water Springerlink

Pdf Electrochemical Removal Of Nitrate Ion From Aqueous Solution By Pulsing Potential Electrolysis

I Love Patterns That S What Made Me Want To Do Etching I Usually Etch Silver In The Tradition Etched Jewelry Metalsmithing Jewelry Jewelry Making Tutorials

Pdf Electrochemical Reduction Of Nitrate Ion On Various Cathodes Reaction Kinetics On Bronze Cathode

Pdf Electrochemical Reduction Of Nitrate Ion On Various Cathodes Reaction Kinetics On Bronze Cathode
Tidak ada komentar