The dihydrate is also three-coordinate with one water coordinated on to the tin and second water coordinated to the first. Not prescribed Protocol Q18 Reaction R 13.

Pdf Synthesis And Characterization Of Ionic Liquids Based Upon 1 Butyl 2 3 Dimethylimidazolium Chloride Zncl2
Stannous chloride is used as a source of tin in radiopharmaceutical kits.

Journal on preparation of tin chloride anhydrous. Strongly heating this salt causes it to decompose into tinIV chloride and potassium chloride. Notes- Preparation of Ferrocene from Anhydrous and Hydrated Ferrous Chloride in Alcohol. International journal of biological macromolecules 162 220-228 2020-06-17.
Present study shows synthesis of Tin Oxide SnO 2 nanoparticles by simple precipitation method at room temperature. Journal of Organometallic Chemistry 1965 3 5 355-363. Triton X-100 and Lauryl alcohol were used as surfactants.
Thermokinetic behaviour of SnCl2 was investigated using differential scanning calorimetry and thermogravimetry techniques under non-isothermal conditions in air complemented by electron microscopy and Raman spectroscopy. This is not a practical procedure for obtaining the anhydrous compound only the pentahydrate. One of the most commonly conducted experiments in the lab is the confirmatory test for stannous ion in TinII Chloride.
Anhydrous tinIV chloride solidifies at 33 C to give monoclinic crystals with the P21c space group. It is commonly used in the Printed Circuit Board PCB industry. According to the results obtained the oxidation of SnCl2 at the heating rates of 5 and 100 C min1 leads to the in situ formation of highly crystalline SnO2.
Anhydrous stannous chloride may be obtained by dehydration of the dihydrate as by drying it under reduced pressure. A range of primary secondary and tertiary alcohols as well as phenolic hydroxyl groups were converted into their corresponding trimethylsilyl ethers with hexamethyldisilazane in the presence of catalytic amounts of silica. It contains a tin 2.
STANNOUS CHLORIDE IS PREPARED. First make a solution of the compound in dilute hydrochloric acid in the ratio 120. Sn 2 Cl 2 SnCl 4 Structure.
TinII chloride anhydrous powder 9999 trace metals basis. It is prepared from reaction of chlorine gas with tin at 115 C 239 F. In the solid state crystalline SnCl 2 anhydrous forms chains linked via chloride bridges as shown in Fig.
Tin II chloride dihydrate not to be confused with Tin II chloride anhydrous is created through dissolving tin in hydrochloric acid. This is followed by evaporation and crystallization. Synthesis of Cyclopentadienyl Metal Compounds.
The dihydrate is made by a similar reaction using hydrochloric acid. Other uses include as a lube oil additive. As a Lewis acid forming complexes with ligands such as chloride ion.
Dec 3 2018 125700 PM. Silica-supported tin chloride SiO 2-SnCl 4-n has been prepared by mixing tin chloride with activated silica gel in toluene under refluxing conditions for one day. The chlorination of domestic tin-plated scrap was studied to determine the potential of this ma- terial as a source of tin for the manufacture of stannous chloride.
Tetrahedron 5145 12277-12284 1995 Tin IV chloride catalyzed cycloaddition reactions between 3-ethoxycyclobutanones and allylsilanes. Submicron tin oxide SnO 2 was obtained from the thermal decomposition of tin oxalate SnC 2 O 4 precipitated at room temperature from mixed solutions of tin II chloride and oxalic acid. The substitution products of tinII dialkylphosphites in 12 molar ratio have been synthesized by reacting benzene solution of sodium dialkylphosphite with ethanolic solution of anhydrous tinII chloride.
Tin IV chloride-promoted synthesis of 4-aminopyridines and 4-aminoquinolines. It is isostructural with SnBr 4. Starting materials were 380 g of benzyl chloride 355 g of tin powder 01 g of mercuric chloride and 300 ml of anhydrous toluene.
Find Sigma-Aldrich-452335 MSDS related peer-reviewed papers technical documents similar products more at Sigma-Aldrich. SnO 2 nanoparticles were synthesized by using hydrated stannic chloride SnCl 45H 2 O as precursor and sodium carbonate as precipitating reagent. Tin II chloride dihydrate then is colorless and crystal-like in appearance and it attracts oxygen from the air.
Filtration cooling washing toluene drying suction Purification. K 2 SnCl 6 2KCl SnCl 4. Equation 1 stoichiometric proportions of tin and iodine toluene and anhydrous calcium chloride auxiliary substances reflux T 100 ºC Isolation I 13.
It has a role as a reducing agent a food additive and a mordant. Oxidation of tinII chloride. Stannous Chloride Anhydrous.
Stannous Chloride Anhydrous or SnCl2 is widely used as a reducing agent in acid solution palladium-tin catalyst and in electrolytic baths for tin-plating. Converted to the chloride 5 by reaction with hydrochloric acid. Tin galvanizing and as a reducing agent in the manufacture of polymers and dyes and printing.
The tinIV chloride boils off and can be condensed. Hydrated tinIV chloride can be prepared by oxidizing tinII chloride in solution using potassium nitrate. The reaction seems to be endothermic in nature and carried out in refluxing benzene 8-9 hrs.
In this study we successfully synthesized nanocrystalline β-SnS 2 at 150C via a solventthermal process which is similar to the well-known hydrothermal process except that toluene is substituted for water. Anhydrous SnCl 2 is prepared by the action of dry hydrogen chloride gas on tin metal. However tin chloride has been found to be very susceptible to water so nanocrystalline β-SnS 2 cannot be synthesized by the hydrothermal method.
The most straightforward synthesis technique and the one that is most used are the direct reaction of tin metal and either chloride or tin tetrachloride or the reaction of hydrogen chloride gas with tin. Veronese A C et al. It is a tin molecular entity and an inorganic chloride.
Sn s 2 HCl aq SnCl 2 aq H 2 g The water then carefully evaporated from the acidic solution to produce crystals of SnCl 2 2H 2 O. Among the more direct methods the preparation of stannous chloride. Synthesis of carbon spheres Glucose 2973 g was dissolved in 300 mL of the mixed solution of deionized water and anhydrous ethanol with a volume ratio of 14 and stirred for 30 min to get a.
All samples were obtained by taking up. SnCl 2 has a lone pair of electrons. Tin reduces technetium-99m the active radiological.
Hydrochloric acid easily dissolves TinII Chloride and therefore the resultant solution can be obtained easily without heating. Tin II chloride anhydrous is an inorganic chloride that has formula Cl2Sn.
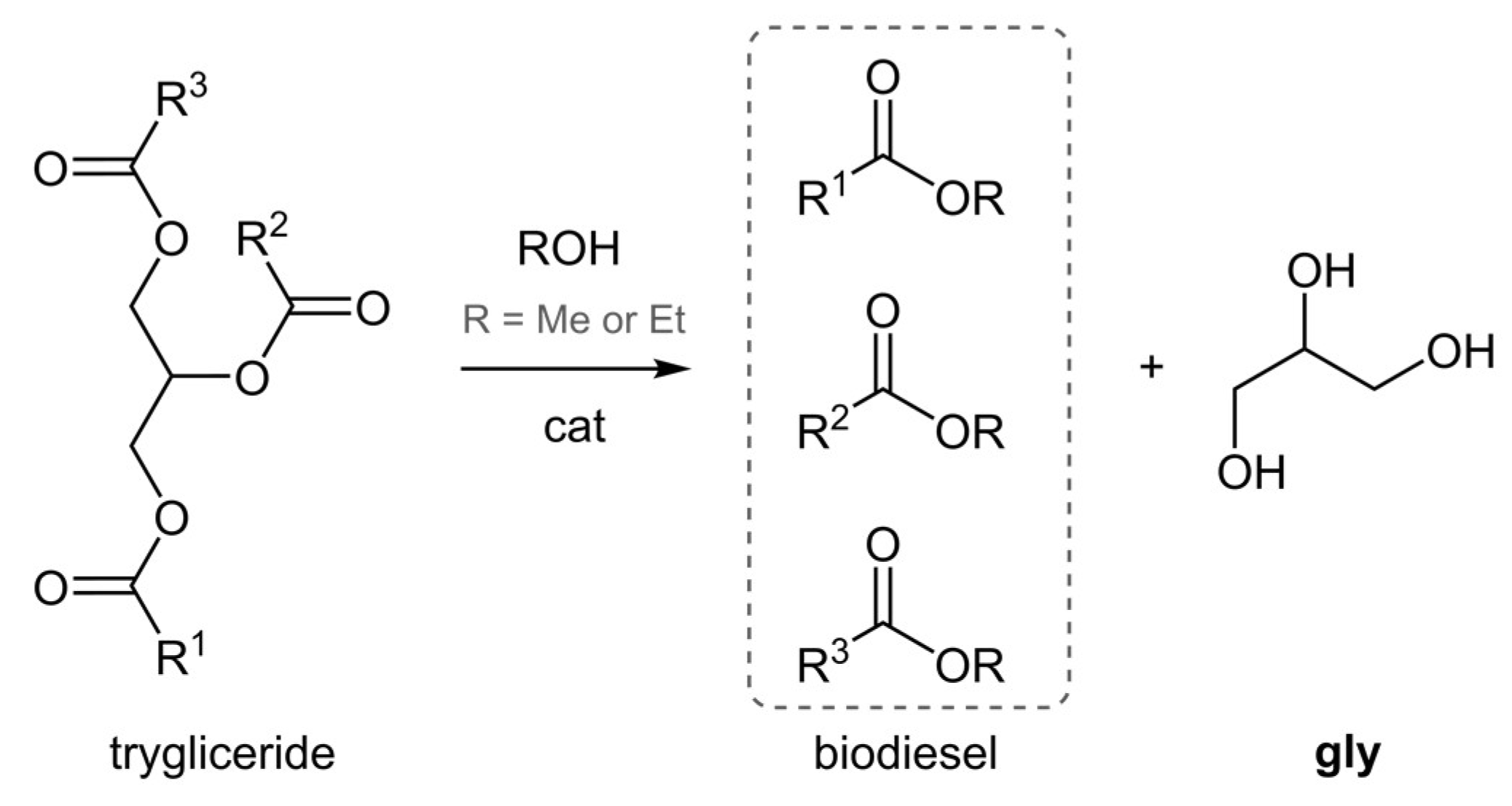
Processes Free Full Text An Expedient Catalytic Process To Obtain Solketal From Biobased Glycerol Html

Stannous Chloride An Overview Sciencedirect Topics
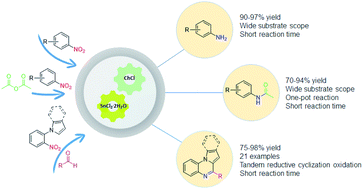
Tin Ii Chloride Dihydrate Choline Chloride Deep Eutectic Solvent Redox Properties In The Fast Synthesis Of N Arylacetamides And Indolo Pyrrolo 1 2 A Quinoxalines Rsc Advances Rsc Publishing

Pdf Synthesis Of Vanadium Diboride Nanoparticles Via Reaction Of Vcl3 With Nabh4

Why Benzyl Chloride Is Highly Reactive In Sn1 Reaction In Spite Of Primary Alkyl Halide Chemsolve Net Reactions Dissociation Green Chemistry
Tidak ada komentar